What Charge Does Co3 Have
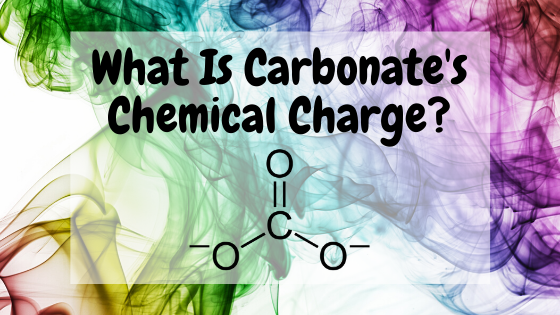
The substance with the chemic formula COiii goes by the proper namecarbonate. Carbonate is fabricated of one atom of carbon and 3 atoms of oxygen and has an electric charge of −ii. This negative charge means that a single ion of carbonate has 2 more electrons than protons.
Carbonate is a flexible polyatomic ion and is characterized by its tendency to grade table salt compounds with alkali and brine earth metals. Carbonate compounds are the primary component of several types of sedimentary rock, the nearly well known beingness limestone which is composed mainly of calcium carbonate (CaCO3). Carbonate compounds also make up the shells of mollusks and coral, as well as in cleaning agents like sodium carbonate (NatwoCOthree) and potassium carbonate (K2CO3). Carbonate compounds are also constitute in the human trunk where they are used equally a buffer to regulate pH levels in the claret.
"Ofttimes referred to as osteoporosis of the bounding main, [ocean acidification] prevents shell edifice creatures such as lobster, oyster, crab, shrimp, and coral from extracting the calcium carbonate from the water that they need to build their shells and are thus unable to survive." — Philippe Cousteau, Jr.
Carbonate is a polyatomic ion, in that it is an ion made from 2 or more than atoms. Commencement, let'southward expect at the general concept of an ion and piece of work up towards the more complex thought of a polyatomic ion.
What Is An Ion?
In a nutshell, an ion is an cantlet that has diff amounts of protons and electrons. All atoms are composed of three kinds of particles, protons, neutrons, and electrons. Protons and neutrons exist bunched together in the nucleus of the atom while electrons be in orbital shells surrounding the nucleus. each particle has an associated electrical charge. Protons take a charge of +ane and electrons accept a charge of −one. Neutrons accept a neutral electric charge of 0.
In a normal atom, at that place exist equal amounts of protons and electrons. In such atoms, the positive charges of the protons and the negative charges of the electrons are exactly equal and contrary, and then the charges cancel out and the atom is overall electrically neutral. This is not always the case though. Atoms can gain or lose electrons, and so have on an overall negative or positive charge. Atoms with not-zippo electrical charges are chosenions.
There are 2 major kinds of ions. Positively charged atoms are calledcations. Cations grade when an atom loses electrons. There are now more protons than electrons so the atom has an overall positive accuse. Negatively charged ions are calledanions and are formed when an atom gains electrons. There are now more electrons than protons and then the atom has an overall negative charge.
"By allowing the positive ions to pass through an electrical field and thus giving them a certain velocity, information technology is possible to distinguish them from the neutral, stationary atoms." — Johannes Stark
Take sodium (Na) every bit an example. A sodium atom has 11 protons and eleven electrons. Sodium has a relatively low ionization energy, pregnant that its electrons can be removed easily. As such, sodium has a tendency tolose electrons and form a positive cation. The electric charge of an ion is commonly written every bit a superscript number next to the chemic symbol. In the example of sodium, sodium unremarkably loses 1 electron so forms an ion with a accuse of +i, written Na+. Fluorine (F), on the other hand, has a high electronegativity and easily picks upwardly actress electrons. An atom of fluorine will pick upward an actress electron to fill its outer shell and makes an ion with a charge of −1, written F−
Ions form bonds via the potent electrostatic attraction between positive and negative ions. In the example of sodium chloride (NaCl), a sodium cation volition bail with a chlorine anion like so:
Ionic bonds tend to exist stronger than covalent bonds due to the stronger electrostatic interaction between ions. Ionic compounds normally are breakable, have high melting/boiling points, and dissolve easily in polar solvents.
Polyatomic Ions
Polyatomic ions, as the proper noun would imply, are ions that are made out of multiple atoms. In other words, a polyatomic ion is just a molecule that has an unequal amount of protons and electrons. Similar monatomic ions, polyatomic ions accept an overall positive or negative charge.
Take ammonium (NHfour +) for case. Ammonium is a polyatomic ion made out of a single nitrogen atom and 4 hydrogen atoms. Ammonium has a full of 9 protons (5 for the nitrogen and 1 for each hydrogen) but just 8 electrons. So, ammonium has an overall accuse of +i. Ammonium can be formed from the protonation (adding a proton) to ammonia (NHthree). The improver of an extra proton gives the unabridged molecule a net positive charge.
Polyatomic ions are most commonly seen in the context of acrid-base chemical reactions. Acidic solutions are formed by dissociating hydrogen atoms from a substance. This process results in free protons (H+ ions) and the corresponding polyatomic conjugate base pair. For example, sulfuric acid (H2SOiv) will dissociate in a solvent to make two H+ ions and its conjugate base, a sulfate polyatomic ion SOiv 2−.
Notice how the sulfate ion keeps ahold of the two electrons originally shared by the hydrogen atoms. The addition of the two electrons from the dissociated hydrogen atoms gives the sulfate ion its overall negative charge of −2.
Ions VS Polarity
Ions and polar molecules are not the aforementioned things. Polar molecules are molecules that have an electric dipole due to the uneven spatial distribution of atoms. Ions are atoms that accept unequal amounts of electrons and protons. Polar compounds comprise covalent bonds, ionic compounds do non. Additionally, polar molecules take partial electric charges while monatomic and polyatomic ions have integer electrical charges.
Carbonate As a Polyatomic Ion
Carbonate is the simplest kind of oxocarbon ion. it is fabricated out of 3 oxygen atoms bonded to a primal carbon atom and has a symmetrical trigonal planar geometry. Carbonate has a molar mass of about sixty g/mol. It is the conjugate base of carbonic acid (H2CO3) and can be made via the dissociation of carbonic acid in a solvent.
The diminutive structure of a carbonate ion cannot exist represented past a single Lewis construction. Common sense would signal that a carbonate cantlet consists of a fundamental carbon cantlet sharing 2 single bonds to negative oxygens and a double bail to a neutral oxygen. Empirical observation indicates that the ion is completely symmetrical and each bond and oxygen atom is equivalent. Thus, carbonate is unremarkably represented by a resonance Lewis construction:

The resonance construction of carbonate. Credit: WikiCommons CC0 1.0
The actual electron structure of a carbonate ion is understood to be some average of these iii separate figures.
Carbonate ions are electrically negative, then have a potent trend to form ionic bonds with positively charged cations. The resulting substance is by and large referred to as a carbonate common salt. In general, carbonate ions form salts with grouping 1 and two alkali and alkaline earth metals. Alkali and alkaline world metals, similar sodium, potassium, calcium, and magnesium, tend to class positive cations so they readily bond with negative carbonate anions. Ane of the most mutual carbonate salts is calcium carbonate (CaCO3). Calcium carbonate is a salt formed by an ionic bail between a calcium cation (Catwo+) and a carbonate anion. Other common carbonate salts include potassium carbonate (K2COiii), magnesium carbonate (MgCOiii) and sodium carbonate (Na2CO3).
Occurrences of Carbonate
Calcium carbonate is a major constituent of most kinds of sedimentary rock. Limestone, for case, is made of mainly calcium carbonate. Limestone can be dissolved by h2o because of its ionic composition. Dissolving limestone in water gives calcium cations and carbonate anions. The deposition of carbonate salts past mineralized water is the main machinery backside the formation of stalactites and stalagmites in caves.

Stalactites are formed from carbonate compounds dissolved in water. Credit: Pixabay CC0 1.0
Carbonate is also an important biological substance. Most obviously, carbonate compounds are excreted by the man body to regulate internal pH levels. For example, when claret pH gets likewise low, meaning that the blood is acidic and has a loftier concentration of hydrogen ions, the body produces carbonate ions. The carbonate ions suck upwardly the excess protons which raise the pH of the claret back to normal levels. When blood pH is too high, the kidneys excrete bicarbonate ions (HCO3 −), which dissociate and innovate more hydrogen ions into the blood. The same mechanism is behind the use of carbonate compounds in antacids. Carbonate ions react and neutralize gastric acid, which eases symptoms of acid reflux and indigestion.
"Indigestion is charged past God with enforcing morality on the tummy." — Victor Hugo
Carbonate compounds likewise play an important part in the germination of atmospheric carbon dioxide. Many marine organisms utilize carbonate buffer systems to regulate their internal pH levels. They exude these carbonate compounds which in turn are converted into carbon dioxide and released into the atmosphere from Earth'due south oceans. Carbonate systems in the oceans are 1 of the primary natural producers of atmospheric carbon dioxide. Increasing sea temperatures tin can upshot in the germination of more carbon dioxide from carbonate compounds dissolved in the ocean, leading to higher concentrations of carbon dioxide in the atmosphere.
In the context of organic chemical science, functional groups that consist of a single carbon and 3 oxygens are often chosen carbonates. Though technically non polyatomic ions, carbonate functional groups retain many of the properties of their freely existing ionic cousins, including their solvent backdrop.
What Charge Does Co3 Have,
Source: https://sciencetrends.com/what-is-the-charge-of-co3/
Posted by: biondohuriturnar.blogspot.com


0 Response to "What Charge Does Co3 Have"
Post a Comment